Oxygen Isotope With 8 Neutrons
One of the many ways in which paleoclimatologists know past climate and ocean conditions is past using the chemical makeup of rock and fossil specimens. Retrieve that chemical elements are composed of some number of protons, neutrons, and electrons. Elements have a charged balance (neither positive or negative) because they take an equal number of electrons and protons. However, various chemical reactions in nature will crusade elements to either gain or lose electrons, and the elements become positively or negatively charged. When this happens, the elements become ions. Positive and negative ions will concenter each to grade solids, some liquids, and some gases. When a solid dissolves in water, the positive and negative ions interruption apart and dissociate through the h2o. Most rocks and fossil-hard parts are fabricated of ionic compounds.
For example table table salt, sodium chloride, will dissolve in water forming the positively-charged sodium ion and negatively-charged chloride ion. This forms an aqueous (a h2o based) solution:
![]()
In the to a higher place equation, the (s) indicates a solid material (tabular array salt), whereas the (aq) indicates that these ions are dissolved in an aqueous solution.
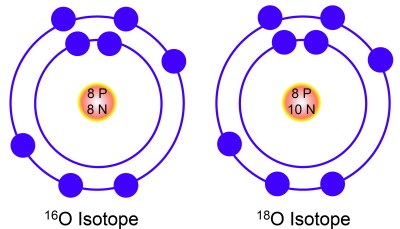
Chemical elements are institute in different versions, called isotopes. Isotopes are elements that contain the aforementioned amount of protons, but differ in the number of neutrons in their nuclei. For case, there are three isotopes of the element oxygen (O): Oxygen 16, 17, and 18. Each isotope of oxygen contains 8 protons, but differs in the number of neutrons. An isotope number is a autograph representation of its mass. Because protons and neutrons are roughly equal in mass, an isotope'southward number is equal to the sum of its protons and neutrons. Therefore, oxygen xvi has eight protons and viii neutrons, oxygen 17 has 8 protons and 9 neutrons, and oxygen xviii has eight protons and 10 neutrons.
There are two primary types of isotopes that geoscientists utilise to translate the aboriginal Earth: stable and unstable isotopes. An unstable isotope experiences radioactive decay, where the chemical element will lose energy over fourth dimension. Several radioactive isotopes occur naturally, and not all are bad or crusade harm to humans. Notwithstanding, paleoclimatologists practise not unremarkably work with these unstable isotopes. Instead, nosotros use stable isotopes that are not undergoing radioactive decay.
Two of the almost common stable isotopes that are used by geoscientists are those of carbon (C) and oxygen (O) . Although there are several types of stable isotopes, we will mainly talk most carbon and oxygen obtained from planktic and benthic foraminifera , as these are very common in paleoclimatology (especially to written report our oceans), but will also briefly touch on other proxies used for isotope analyses.
How are carbon and oxygen isotopes obtained?

Paleoclimatologists obtain carbon and oxygen isotopes from calcite , a common diversity of calcium carbonate, with the chemical formula CaCO iii . In this formula, there are three elements: calcium (Ca), carbon (C), and three oxygen atoms (O). Calcite and calcium carbonate are mutual on the Earth and in the oceans, and can accept several forms. Here we will talk briefly about the most common types of calcite used for isotope analysis.
Calcite is a component in many sedimentary rocks . When a sedimentary stone is equanimous dominantly of calcium carbonate, geoscientists call it a limestone . Limestone rocks are easy to erode compared metamorphic and igneous rocks. Calcium carbonate dissolves when exposed to acids. Because rainwater is slightly acidic, prolonged exposure to rain will chemically erode abroad limestone rock formations (or even a limestone statue for that matter).

When this occurs, the dissolved ions from limestone are then carried by water into the soil, where they can eventually notice their way to caves. Here, the limestone ions take space to drip into the cavern and course new limestone formations in the class of stalactites and stalagmites (commonly referred to as speleothems ). To analyze stable isotopes of carbon and oxygen from speleothems, they are cutting out of a cave and taken to a lab, where they are sawed in half and polished. A microdrill is then used to drill tiny samples from defined intervals along the speleothem for isotope analysis.
Calcite is also used by marine organisms to build their shells and hard parts. Invertebrate animals (those lacking a backbone) take been using dissolved calcite ions to build their shells since at least the Cambrian (~550 1000000 years ago). Mutual fossil groups that utilize calcite include brachiopods, trilobites, and ancient echinoderms, such as blastoids . Some extant (however living) animals, similar sea urchins and oysters also build their skeletons from calcite. In improver, some protists, such as planktic and benthic foraminifera , use calcite to build their tests. Calcite-producing organisms record the values of carbon and oxygen in their shells, and tin can be analyzed for carbon and oxygen isotopes.
In rocks of Paleozoic age, scientists unremarkably obtain oxygen isotopes from another blazon of fossil: conodonts . These small, tooth-like fossils are all that remain of ancient eel-like organisms that represent some of the earliest chordates. Conodonts are commonly found in limestone rocks as these creatures swam in the seas in which the limestone was deposited. Unlike the calcareous brachiopods and trilobites that they lived among, conodont teeth are made of apatite, or calcium phosphate, with the chemical formula Ca3O8Ptwo. These scientists can analyze conodonts to obtain oxygen isotopes.
Scientists can as well utilize limestone samples taken directly from an outcrop to analyze isotopes of carbon and oxygen. Obtaining these bulk carbonate samples of limestone typically involves finding a suitable outcrop of limestone, hammering away some chunks at defined intervals, and taking the samples back to the lab to clarify.
How are carbon and oxygen isotopes measured?

Once the advisable material (limestone samples, speleothems, or fossils) is collected for isotope analyses, a small sample is put into a mass spectrometer to measure the amounts of carbon and oxygen isotopes within each sample. Each sample is loaded into a vial, and all the vials are and then put into a carousel (see image at left, with red arrow pointing to sample carousel). Approximately three drops of acid are put into the vials to dissolve the sample, creating a gas that contains the ions to be measured. Ions are very reactive, so the measurements within the mass spectrometer take place within a vacuum. There are several different types of mass spectrometers, only ane of the common ways to measure isotopes is to dispense them by magnets and electric fields, and shoot them down a bent tube.
Considering isotopes of elements differ in weight due to additional neutrons (for instance, oxygen with eighteen neutrons is heavier than an oxygen molecule with sixteen neutrons), they will deflect at unlike angles in the tube. The caste to which the ions/atoms are deflected by a magnet is how heavy they are. A heavier ion/cantlet/molecule is harder for the magnet to deflect, so it will only plough slightly, while a lighter i/a/m has less inertia and is easier to turn.
Thus, lighter molecules are deflected more than heavier ones. This information is sent to a calculator, which gives the researcher data on the amount of each isotope in every sample.
For a more than detailed account of how mass spectrometry works, click here . For a video demonstration on how ions are deflected within a mass spectrometer, c lick here .
To larn how paleoclimatologists translate carbon and oxygen isotopes, go on to the 'Carbon & Oxygen Isotopes' page!
Oxygen Isotope With 8 Neutrons,
Source: https://timescavengers.blog/introductory-material/what-is-paleoclimatology/isotopes/
Posted by: williamsstenly.blogspot.com


0 Response to "Oxygen Isotope With 8 Neutrons"
Post a Comment